A study just published by TIGEM researchers shows how the efficacy of this experimental therapy is maintained over time, at the highest doses. Study coordinator Brunetti-Pierri reports on possible future developments
Seven years have passed since the day that Nicola Brunetti-Pierri, a physician and researcher at the Telethon Institute of Genetics and Medicine (Tigem) at the 'Federico II' university polyclinic in Naples, administered the experimental gene therapy developed in the institute's laboratories in Pozzuoli to a patient with mucopolysaccharidosis type 6 (MPS6) for the first time.
This rare genetic disease is due to the deficiency of an enzyme, arylsulphatase B (ARSB), which leads to the accumulation in the body of certain sugars called glycosaminoglycans: from childhood onwards, the accumulation of these substances causes skeletal deformities and short stature, thickening of certain heart valves, enlargement of the liver and spleen, and opacity of the cornea. An enzyme replacement therapy is currently available, but it has major limitations: to try to overcome them, Tigem's current director Alberto Auricchio and his group have developed a strategy that uses vectors of viral origin to supply the liver with a corrected version of the gene coding for the ARSB enzyme, as illustrated in the diagram below.
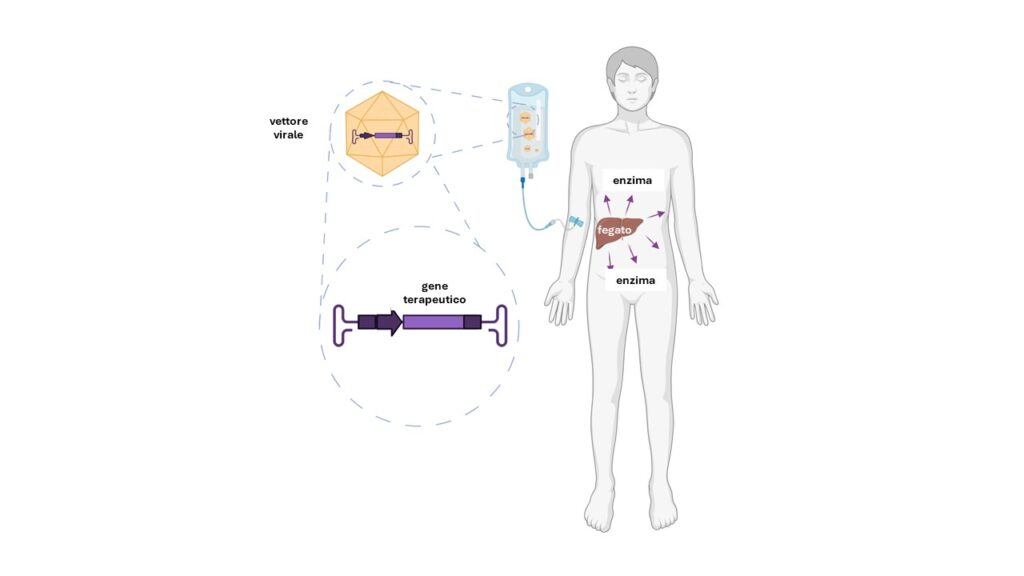
To date, nine patients, from Italy but also from Canada, Turkey and Spain, have received the experimental treatment: in an article just published in the scientific journal Med-Cell Press, clinical trial coordinator Nicola Brunetti-Pierri takes stock of the situation.
'Our gene therapy protocol was the first - and still the only one - to be tested in humans for this disease. As with all 'firsts', one has to start by assessing safety: we therefore began by administering low doses of the viral vector. Encouraged by the fact that no harmful effects were observed in the first patients treated, we gradually increased the dose: those described in this paper are the effects of the gene therapy observed about four years later in the four patients who received the highest dose of the drug: Alba, Violet, Jasper and Yvonne. In all of them, we observed satisfactory production of the enzyme, just enough so that we did not have to resume the periodic infusion as was the case before the gene therapy. Only in one case did we decide to restart it as a precaution because we detected above normal levels of glycosaminoglycans in the urine. Moreover, all of them maintained stable motor capacity as well as pulmonary and cardiac functions: comforting data, as these are among the key organs that can be damaged over time by the disease'.
As in all clinical trials, observing the effects over time teaches us a lot and allows us to think about how the therapeutic strategy can be improved. In this sense, the research work within the AAVolution European project, coordinated by Tigem, which aims to improve and expand the clinical applications of innovative gene therapy strategies directed at the liver and based on adeno-associated viral vectors (AAV), such as the one being tested for MPS6, will be very important.
'One of the aspects we can improve is the duration of the therapeutic effect,' Brunetti-Pierri explains. AAV-type vectors, once they have entered the cells, do not integrate into the DNA, as lentiviral vectors derived from HIV do. This avoids the possible risks of integration at critical points in the genome, but at the same time can lead to a loss of therapeutic effect over time: when cells divide, especially during growth or even in adult life to repair damage, the vector is not transmitted to daughter cells, which will therefore no longer be able to produce the missing enzyme. We are therefore investigating how to overcome this problem by exploiting CRISPR-Cas9-based gene editing technology: instead of inserting the correct copy of the gene into the vector, we could provide the 'molecular scalpel' capable of correcting the genetic defect directly on the DNA of the liver cells, in a definitive way'.
In the meantime, thanks to the support of the Telethon Foundation, the trial will resume in 2026 with the treatment of new patients at a higher dose of the viral vector, with the hope of observing an even greater therapeutic effect. 'Another possibility could be to repeat the administration, if we manage to "trick" the immune system,' the researcher continues. Another obstacle in patients who have already received the vector is in fact the presence in the blood of antibodies that neutralize the vector itself, as if it were a pathogen. Also, as part of the AAVolution project, we are studying how to reduce the levels of these antibodies transiently, only close to the administration of the vector: this would also be very useful for all gene therapies based on these vectors for other diseases, since the presence of neutralizing antibodies has been found in about a third of patients'.


